|
 Overview | Understanding Coagulation | Assessment & Monitoring | Clinical Contexts | Treatment Strategies | Reversal of Anticoagulation & Antiplatelets | Guidelines & Evidence Overview | Understanding Coagulation | Assessment & Monitoring | Clinical Contexts | Treatment Strategies | Reversal of Anticoagulation & Antiplatelets | Guidelines & Evidence
Pharmacologic Alternatives: Factor Concentrates
Contributed by: Jacob Raphael, MD, FAHA

Inherited and acquired coagulopathy are frequently associated with major bleeding in severe trauma, cardiac and major non-cardiac surgery and postpartum hemorrhage. Additionally, due to increase in the use of anticoagulants and anti-platelet medications, significant bleeding may also occur in non-surgical patients and following minor trauma. A large body of evidence associates bleeding and transfusion of blood products with increased morbidity and mortality as well as prolonged hospital stay and increased health care costs.1,2 Coagulopathy and bleeding may be multifactorial and can be exacerbated by hypothermia, acidosis, hypovolemia, hemodilution, thrombocytopenia, coagulation factor deficiency and fibrinolysis.3 Over the last decade targeted goal-directed therapy using viscoelastic point-of-care testing and coagulation factor concentrates as alternatives to pro-hemostatic allogeneic blood products, are increasingly used to guide hemostatic therapy. Guidelines for perioperative bleeding report specific management algorithms and particularly emphasize the use of targeted hemostatic resuscitation with coagulation factor concentrates.4-7
Prothrombin Complex Concentrate (PCC)
PCCs are coagulation factor concentrates that are purified from plasma and include the vitamin K dependent coagulation factors II, IX, and X (in 3-factor PCCs) as well as Factor VII (in 4-factor PCCs) and various concentrations of heparin, antithrombin and proteins C and S to avoid excessive thrombosis.8 The development of PCCs emerged from the search for a purified factor IX concentrate to treat hemophilia B 9. As such, each factor within individual formulation is communicated as international units (IU) per 100 IU of Factor IX. Furthermore, the total dose administered is also conveyed using this standard language of IU of Factor IX (i.e., 1000 IU of PCCs means 1000 IU of Factor IX). Clinically available PCCs are routinely defined as activated or non-activated. Activated PCCs were developed for the treatment of hemophilia patients who have antibodies to factor VIII or IX, therefore, “bypassing agents” were introduced to enhance Factor Xa production via the extrinsic Xase complex of tissue factor (TF) and Factor VIIa, thereby restoring thrombin generation and hemostasis. Factor Eight Inhibitor Bypassing Activity (FEIBA®, Baxter Healthcare, Bloomington, Indiana, USA) is the only available activated PCC in the United States, containing varying levels of Factors VII (including appreciable amounts of Factor VIIa), II, IX, and X (including very small amounts of factor Xa). Non activated PCCs include 3-Factor (containing factors II, IX, and X) or 4-Factor (containing factors II, VII, IX, and X) formulations. Table 1 shows various commonly clinically-available PCC formulation.
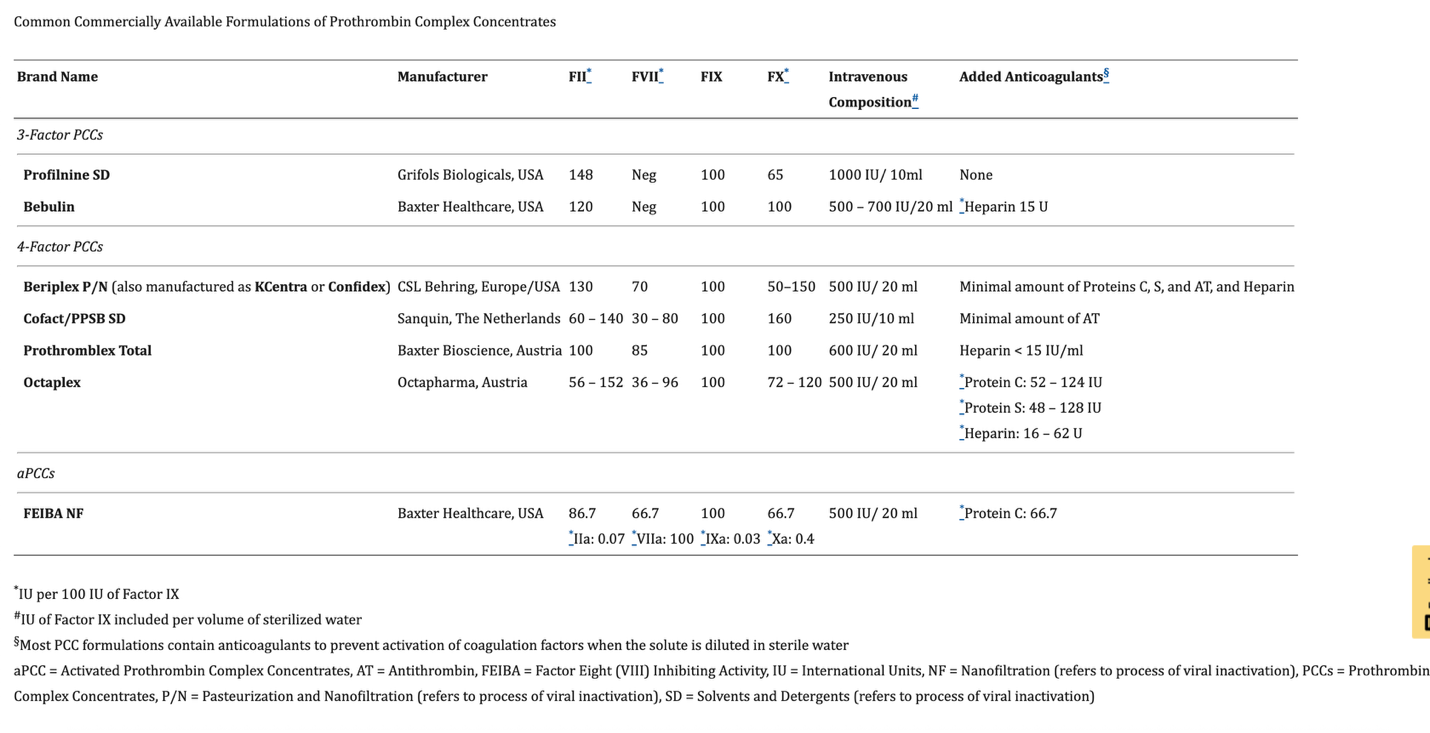
While providing non-inferior (or maybe even superior) hemostatic efficacy 10, PCC use offers several advantages, in comparison to plasma, including: no need for type and screen or other processing steps in the blood bank, longer shelf life, a significantly smaller volume of administration, which is of particular importance in patients who require volume restriction such as in subjects with heart failure, shorter infusion times 11 and enhanced safety profile due to additional pathogen removal steps such as nanofiltration and viral inactivation. In addition, while initially, concerns regarding increased risk for thromboembolic complications have been raised 12, a large pharmacovigilance investigation reported that the use of PCC was not associated with increased risk of thromboembolic complications when compared to FFP 13.
PCC use for anticoagulation reversal
In the USA, Non-activated, 4F-PCCs are only FDA-approved for the urgent reversal of vitamin K antagonists (VKA) in adults with acute major bleeding or the need for urgent/emergent surgery 14. A meta-analysis of 12 studies with 1597 subjects showed that the four-factor PCCs are more effective when used for this indication than the three-factor PCCs 15. With the increased use of DOACs, PCCs are now also considered as a reasonable (yet, off-label) alternative to treat the coagulopathy induced by those agents in trauma and surgical patients, especially when Andexanet alpha is contraindicated or unavailable 16, 17.Furthermore, current recommendations of the American Heart Association and American Stroke Association indicate that four-factor PCC rather than FFP is recommended as first-line therapy in anticoagulated patients with spontaneous intracerebral hemorrhage 18.
PCC use in acquired perioperative coagulopathy and bleeding
A large body of information on the use of PCC in the perioperative setup derives from cardiac surgery. In cardiac surgery, the use of PCCs to treat post-CPB coagulopathy has increased significantly over the last decade, and although still defined as off-label use in the USA, it is recommended as an alternative to plasma in recent published guidelines 6,19. Several small prospective trials have shown some benefit in reducing RBC transfusions when treating patients with post-CPB bleeding with PCCs 20,21. The recently published Prothrombin Complex Concentrate vs Frozen Plasma for Coagulopathic Bleeding in Cardiac Surgery (FARES II) trial 10 was a multicenter unblinded randomized non-inferiority-controlled trial that compared hemostatic effectiveness between four-factor PCC and frozen plasma in 538 patients undergoing cardiac surgery with coagulopathic bleeding. In this trial, PCC was found to have better hemostatic effectiveness, with less allogeneic blood transfusions, and fewer adverse events including lower incidence of postoperative AKI as compared to frozen plasma. While the findings of this trial indicate that PCCs are most probably superior to plasma in treating coagulopathic bleeding after CPB, several experts have raised concerns regarding overdosing of PCC compared to FFP and the exclusion of patients with recent myocardial infarctions from the investigation 22. The 4-RESTORE trial, a multicenter phase-3 RCT that compares the use of PCC to FFP in bleeding cardiac surgery patients with evidence of coagulation factor deficiency, is currently recruiting subjects to further assess the efficacy of PCCs in treating bleeding cardiac surgery patients in comparison to FFP (NCT04244981).
Comparing PCC with activated factor VII
Before PCCs have gained their increased popularity in treating acquired coagulopathy and bleeding, multiple publications reported the off-label use of recombinant activated factor VII in bleeding unresponsive to other hemostatic therapy. Although proved to be effective in post-CPB coagulopathic bleeding, most studies reporting its use are retrospective observational reports of patients having received multiple transfusions and other therapeutic agents for refractory bleeding 23. A prospective analysis of 4,468 non-hemophilia subjects (4,119 patients and 349 healthy volunteers), arterial thromboembolic events in patients treated with factor VIIa patients were significantly higher compared to placebo particularly in patients older than 65 24. In comparison to patients with hemophilia, the recommend dose of factor VIIa to treat post-CPB coagulopathic bleeding is significantly lower, with Recent observational studies indicating that a lower dose of factor VIIa of approximately 13 µg/kg is effective in reducing bleeding without being associated with increased thromboembolic or renal complications 25. When directly comparing the use of PCCs vs. factor VIIa harper et al 26 using propensity score matching analysis reported that activator factor VIIa was associated with a higher volume of postoperative bleeding, higher volume of RBC transfusion, and higher percentage of patients requiring additional plasma, platelets and cryoprecipitate supplementation. In addition, patients who received factor VIIa had a higher rate of postoperative morbidity compared to those who received PCC. Furthermore, in a recent propensity score matched analysis in children undergoing surgery for congenital heart disease, the incidence of thrombotic complications was significantly lower in patients receiving 4-factor PCC compared to those who received factor VIIa.27
Fibrinogen Concentrate
Fibrinogen, a critical hemostatic protein that is synthesized by the liver, is often the initial factor to reach critically low levels and requires repletion as part of resuscitation 28. Proposed mechanisms include coagulation activation–induced consumption, degradation by hyperfibrinolysis, and dilution by volume replacement. Normal fibrinogen levels are between 200-400 mg/dl, whereas in pregnancy fibrinogen level increase significantly.29 Thus, fibrinogen supplementation plays a crucial role in postpartum hemorrhage. In cardiac surgery observational studies have reported an association between lower pre and postoperative fibrinogen levels and higher risk for post-CPB bleeding.30,31
Older guidelines suggested a transfusion trigger of 100 mg/dl of fibrinogen, while current European guidelines recommended levels of greater than 150-200 mg/dl. To achieve the target fibrinogen levels, 3 to 4g fibrinogen concentrate, or 15 to 20 units cryoprecipitate, are recommended in the bleeding patient5,19.
Fibrinogen concentrate as an alternative to cryoprecipitate
Fibrinogen concentrate is increasingly used as an alternative to cryoprecipitate, especially when fibrinogen supplementation is needed in post-CPB bleeding. Although both products are plasma-derived, they have distinct features: Cryoprecipitate is a non-purified product, hence, in addition to fibrinogen it contains fibronectin and platelet microparticles, as well as coagulation factors VIII, XIII, and von Willebrand factor. Its fibrinogen content varies widely (from 3-30 g/L per unit). It is stored in a frozen state and then thawed and pooled (typically 5-10 unit pools) before administration, and it has a limited shelf life after thawing (4-6 hours). Fibrinogen concentrates are pathogen-reduced and purified, have standardized fibrinogen content (20 g/L), are lyophilized, allowing for easy storage, reconstitution, and administration; and have longer shelf life after reconstitution (up to 24 hours), which reduces wastage. Cryoprecipitate remains the therapy of choice in many countries (including in the USA). In many European countries, however, fibrinogen concentrates have replaced cryoprecipitate.
Initial studies assessing the use of fibrinogen concentrate in cardiac surgery patients where somewhat conflicting. In a small randomized controlled single-center trial in 61 patients undergoing major thoracic or thoracoabdominal aortic surgery, transfusion of fibrinogen concentrate to a maximum clot firmness of 22 mm in the functional fibrinogen assay of the ROTEM was compared with placebo 32. In the fibrinogen arm, the mean transfusion rate after administration of a median dose of 8 g fibrinogen was 2 units versus 13 units in the placebo arm (P < 0.001). In another randomized controlled single-center trial of 116 high-risk cardiac surgery patients, first-line fibrinogen supplementation with the same high fibrinogen threshold was compared to placebo. Fibrinogen supplementation with a median dose of 4 g resulted in a significantly lower primary endpoint of blood product transfusion rate and postoperative bleeding. Of note, only in the treatment arm could patients receive four-factor PCC when the coagulation time in the EXTEM assay of ROTEM was less than 80 s.33 Opposing results however were reported in the large, randomized evaluation of fibrinogen versus placebo in the complex cardiovascular surgery (REPLACE) trial which enrolled 519 patients from 34 centers 34. In this study, fibrinogen concentrate was associated with an unexpected increase in allogenic blood transfusions compared to placebo. In another single-center randomized controlled trial in 120 high-risk cardiac surgery patients, there was no significant difference in the primary outcome of intraoperative blood loss when fibrinogen concentrations exceeded 2.5 mg/dl using fibrinogen concentrate compared with placebo.35 The recently published FIBRES trial36provided strong evidence that fibrinogen concentrate is non-inferior and, in many cases, superior to cryoprecipitate in treating post-CPB bleeding that is associated with hypofibrinogenemia. In this large randomized cardiac surgery clinical trial, patients with clinically significant bleeding and hypofibrinogenemia after cardiac surgery were randomized to receive either 4 g fibrinogen concentrate or 10 U cryoprecipitate within 24 h after CPB. The study confirmed that fibrinogen concentrate was a safe and an effective alternative to cryoprecipitate for fibrinogen repletion. As such, the use of fibrinogen concentrate is recommended as an accepted alternative to cryoprecipitate in recently published guidelines 6,19.
Although fibrinogen concentrate has been studied in the context of postpartum hemorrhage and trauma37-40, there is currently a lack of robust evidence to provide strong recommendations regarding the use of fibrinogen concentrate as first line for fibrinogen supplementation in perioperative bleeding situations outside of cardiac surgery, and further investigation is needed.
References
- Dyke C, Aronson S, Dietrich W, Hofmann A, Karkouti K, Levi M, Murphy GJ, Sellke FW, Shore-Lesserson L, von Heymann C, et al. Universal definition of perioperative bleeding in adult cardiac surgery. J Thorac Cardiovasc Surg. 2014;147:1458-1463 e1451. doi: 10.1016/j.jtcvs.2013.10.070
- Ranucci M, Baryshnikova E, Castelvecchio S, Pelissero G, Surgical, Clinical Outcome Research G. Major bleeding, transfusions, and anemia: the deadly triad of cardiac surgery. Ann Thorac Surg. 2013;96:478-485. doi: 10.1016/j.athoracsur.2013.03.015
- Levy JH, Dutton RP, Hemphill JC, 3rd, Shander A, Cooper D, Paidas MJ, Kessler CM, Holcomb JB, Lawson JH, Hemostasis Summit P. Multidisciplinary approach to the challenge of hemostasis. Anesth Analg. 2010;110:354-364. doi: 10.1213/ANE.0b013e3181c84ba5
- Collins P, Abdul-Kadir R, Thachil J, Subcommittees on Women' s Health Issues in T, Haemostasis, on Disseminated Intravascular C. Management of coagulopathy associated with postpartum hemorrhage: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14:205-210. doi: 10.1111/jth.13174
- Kietaibl S, Ahmed A, Afshari A, Albaladejo P, Aldecoa C, Barauskas G, De Robertis E, Faraoni D, Filipescu DC, Fries D, et al. Management of severe peri-operative bleeding: Guidelines from the European Society of Anaesthesiology and Intensive Care: Second update 2022. Eur J Anaesthesiol. 2023;40:226-304. doi: 10.1097/EJA.0000000000001803
- Tibi P, McClure RS, Huang J, Baker RA, Fitzgerald D, Mazer CD, Stone M, Chu D, Stammers AH, Dickinson T, et al. STS/SCA/AmSECT/SABM Update to the Clinical Practice Guidelines on Patient Blood Management. Ann Thorac Surg. 2021;112:981-1004. doi: 10.1016/j.athoracsur.2021.03.033
- Vlaar APJ, Dionne JC, de Bruin S, Wijnberge M, Raasveld SJ, van Baarle F, Antonelli M, Aubron C, Duranteau J, Juffermans NP, et al. Transfusion strategies in bleeding critically ill adults: a clinical practice guideline from the European Society of Intensive Care Medicine. Intensive Care Med. 2021;47:1368-1392. doi: 10.1007/s00134-021-06531-x
- Ghadimi K, Levy JH, Welsby IJ. Prothrombin Complex Concentrates for Bleeding in the Perioperative Setting. Anesth Analg. 2016;122:1287-1300. doi: 10.1213/ANE.0000000000001188
- Key NS, Negrier C. Coagulation factor concentrates: past, present, and future. Lancet. 2007;370:439-448. doi: 10.1016/S0140-6736(07)61199-4
- Karkouti K, Callum JL, Bartoszko J, Tanaka KA, Knaub S, Brar S, Ghadimi K, Rochon A, Mullane D, Couture EJ, et al. Prothrombin Complex Concentrate vs Frozen Plasma for Coagulopathic Bleeding in Cardiac Surgery: The FARES-II Multicenter Randomized Clinical Trial. JAMA. 2025;333:1781-1792. doi: 10.1001/jama.2025.3501
- Goldstein JN, Refaai MA, Milling TJ, Jr., Lewis B, Goldberg-Alberts R, Hug BA, Sarode R. Four-factor prothrombin complex concentrate versus plasma for rapid vitamin K antagonist reversal in patients needing urgent surgical or invasive interventions: a phase 3b, open-label, non-inferiority, randomised trial. Lancet. 2015;385:2077-2087. doi: 10.1016/S0140-6736(14)61685-8
- Cappabianca G, Mariscalco G, Biancari F, Maselli D, Papesso F, Cottini M, Crosta S, Banescu S, Ahmed AB, Beghi C. Safety and efficacy of prothrombin complex concentrate as first-line treatment in bleeding after cardiac surgery. Crit Care. 2016;20:5. doi: 10.1186/s13054-015-1172-6
- Milling TJ, Voronov A, Schmidt DS, Lindhoff-Last E. Long-Term Safety of a Four-Factor Prothrombin Complex Concentrate (Kcentra(R)/Beriplex(R) P/N): An Updated Pharmacovigilance Review. Thromb Haemost. 2025;125:46-57. doi: 10.1055/s-0044-1788305
- Sarode R, Milling TJ, Jr., Refaai MA, Mangione A, Schneider A, Durn BL, Goldstein JN. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation. 2013;128:1234-1243. doi: 10.1161/CIRCULATIONAHA.113.002283
- Margraf DJ, Brown SJ, Blue HL, Bezdicek TL, Wolfson J, Chapman SA. Comparison of 3-factor versus 4-factor prothrombin complex concentrate for emergent warfarin reversal: a systematic review and meta-analysis. BMC Emerg Med. 2022;22:14. doi: 10.1186/s12873-022-00568-x
- Rayatdoost F, Braunschweig T, Maron B, Schochl H, Akman N, Rossaint R, Herzog E, Heitmeier S, Grottke O. Reversing Rivaroxaban Anticoagulation as Part of a Multimodal Hemostatic Intervention in a Polytrauma Animal Model. Anesthesiology. 2021;135:673-685. doi: 10.1097/ALN.0000000000003899
- Hoffman M, Goldstein JN, Levy JH. The impact of prothrombin complex concentrates when treating DOAC-associated bleeding: a review. Int J Emerg Med. 2018;11:55. doi: 10.1186/s12245-018-0215-6
- Greenberg SM, Ziai WC, Cordonnier C, Dowlatshahi D, Francis B, Goldstein JN, Hemphill JC, 3rd, Johnson R, Keigher KM, Mack WJ, et al. 2022 Guideline for the Management of Patients With Spontaneous Intracerebral Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2022;53:e282-e361. doi: 10.1161/STR.0000000000000407
- Casselman FPA, Lance MD, Ahmed A, Ascari A, Blanco-Morillo J, Bolliger D, Eid M, Erdoes G, Haumann RG, Jeppsson A, et al. 2024 EACTS/EACTAIC Guidelines on patient blood management in adult cardiac surgery in collaboration with EBCP. Eur J Cardiothorac Surg. 2025;67. doi: 10.1093/ejcts/ezae352
- Karkouti K, Bartoszko J, Grewal D, Bingley C, Armali C, Carroll J, Hucke HP, Kron A, McCluskey SA, Rao V, et al. Comparison of 4-Factor Prothrombin Complex Concentrate With Frozen Plasma for Management of Hemorrhage During and After Cardiac Surgery: A Randomized Pilot Trial. JAMA Netw Open. 2021;4:e213936. doi: 10.1001/jamanetworkopen.2021.3936
- Smith MM, Schroeder DR, Nelson JA, Mauermann WJ, Welsby IJ, Pochettino A, Montonye BL, Assawakawintip C, Nuttall GA. Prothrombin Complex Concentrate vs Plasma for Post-Cardiopulmonary Bypass Coagulopathy and Bleeding: A Randomized Clinical Trial. JAMA Surg. 2022;157:757-764. doi: 10.1001/jamasurg.2022.2235
- Mazzeffi M, Henderson R. Questions About Strategies and Dosing Levels of PCC for Coagulopathic Bleeding in Cardiac Surgery. JAMA. 2025;334:831. doi: 10.1001/jama.2025.8991
- Simpson E, Lin Y, Stanworth S, Birchall J, Doree C, Hyde C. Recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia. Cochrane Database Syst Rev. 2012;2012:CD005011. doi: 10.1002/14651858.CD005011.pub4
- Levi M, Levy JH, Andersen HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med. 2010;363:1791-1800. doi: 10.1056/NEJMoa1006221
- Sutherland L, Houchin A, Wang T, Wang S, Moitra V, Sharma A, Zorn T, 3rd, Flynn BC. Impact of Early, Low-Dose Factor VIIa on Subsequent Transfusions and Length of Stay in Cardiac Surgery. J Cardiothorac Vasc Anesth. 2022;36:147-154. doi: 10.1053/j.jvca.2021.04.043
- Harper PC, Smith MM, Brinkman NJ, Passe MA, Schroeder DR, Said SM, Nuttall GA, Oliver WC, Barbara DW. Outcomes Following Three-Factor Inactive Prothrombin Complex Concentrate Versus Recombinant Activated Factor VII Administration During Cardiac Surgery. J Cardiothorac Vasc Anesth. 2018;32:151-157. doi: 10.1053/j.jvca.2017.07.011
- Faraoni D, Guindi A, Ankola AA, Resheidat A, Binsalamah Z, Teruya J, Savorgnan F, Vener DF. Retrospective Comparison of Recombinant Activated Factor VII Versus 4-Factor Prothrombin Complex Concentrate in Cardiac Surgical Patients. J Cardiothorac Vasc Anesth. 2024;38:388-393. doi: 10.1053/j.jvca.2023.11.035
- Hiippala ST, Myllyla GJ, Vahtera EM. Hemostatic factors and replacement of major blood loss with plasma-poor red cell concentrates. Anesth Analg. 1995;81:360-365. doi: 10.1097/00000539-199508000-00026
- Vermeulen T, Van de Velde M. The role of fibrinogen in postpartum hemorrhage. Best Pract Res Clin Anaesthesiol. 2022;36:399-410. doi: 10.1016/j.bpa.2022.10.002
- Walden K, Jeppsson A, Nasic S, Backlund E, Karlsson M. Low preoperative fibrinogen plasma concentration is associated with excessive bleeding after cardiac operations. Ann Thorac Surg. 2014;97:1199-1206. doi: 10.1016/j.athoracsur.2013.11.064
- Karkouti K, Callum J, Crowther MA, McCluskey SA, Pendergrast J, Tait G, Yau TM, Beattie WS. The relationship between fibrinogen levels after cardiopulmonary bypass and large volume red cell transfusion in cardiac surgery: an observational study. Anesth Analg. 2013;117:14-22. doi: 10.1213/ANE.0b013e318292efa4
- Rahe-Meyer N, Solomon C, Hanke A, Schmidt DS, Knoerzer D, Hochleitner G, Sorensen B, Hagl C, Pichlmaier M. Effects of fibrinogen concentrate as first-line therapy during major aortic replacement surgery: a randomized, placebo-controlled trial. Anesthesiology. 2013;118:40-50. doi: 10.1097/ALN.0b013e3182715d4d
- Ranucci M, Baryshnikova E, Crapelli GB, Rahe-Meyer N, Menicanti L, Frigiola A, Surgical Clinical Outcome RG. Randomized, double-blinded, placebo-controlled trial of fibrinogen concentrate supplementation after complex cardiac surgery. J Am Heart Assoc. 2015;4:e002066. doi: 10.1161/JAHA.115.002066
- Rahe-Meyer N, Levy JH, Mazer CD, Schramko A, Klein AA, Brat R, Okita Y, Ueda Y, Schmidt DS, Ranganath R, et al. Randomized evaluation of fibrinogen vs placebo in complex cardiovascular surgery (REPLACE): a double-blind phase III study of haemostatic therapy. Br J Anaesth. 2016;117:41-51. doi: 10.1093/bja/aew169
- Bilecen S, de Groot JA, Kalkman CJ, Spanjersberg AJ, Brandon Bravo Bruinsma GJ, Moons KG, Nierich AP. Effect of Fibrinogen Concentrate on Intraoperative Blood Loss Among Patients With Intraoperative Bleeding During High-Risk Cardiac Surgery: A Randomized Clinical Trial. JAMA. 2017;317:738-747. doi: 10.1001/jama.2016.21037
- Callum J, Farkouh ME, Scales DC, Heddle NM, Crowther M, Rao V, Hucke HP, Carroll J, Grewal D, Brar S, et al. Effect of Fibrinogen Concentrate vs Cryoprecipitate on Blood Component Transfusion After Cardiac Surgery: The FIBRES Randomized Clinical Trial. JAMA. 2019;322:1966-1976. doi: 10.1001/jama.2019.17312
- Zaidi A, Kohli R, Daru J, Estcourt L, Khan KS, Thangaratinam S, Green L. Early Use of Fibrinogen Replacement Therapy in Postpartum Hemorrhage-A Systematic Review. Transfus Med Rev. 2020;34:101-107. doi: 10.1016/j.tmrv.2019.12.002
- Ducloy-Bouthors AS, Mercier FJ, Grouin JM, Bayoumeu F, Corouge J, Le Gouez A, Rackelboom T, Broisin F, Vial F, Luzi A, et al. Early and systematic administration of fibrinogen concentrate in postpartum haemorrhage following vaginal delivery: the FIDEL randomised controlled trial. BJOG. 2021;128:1814-1823. doi: 10.1111/1471-0528.16699
- Innerhofer P, Fries D, Mittermayr M, Innerhofer N, von Langen D, Hell T, Gruber G, Schmid S, Friesenecker B, Lorenz IH, et al. Reversal of trauma-induced coagulopathy using first-line coagulation factor concentrates or fresh frozen plasma (RETIC): a single-centre, parallel-group, open-label, randomised trial. Lancet Haematol. 2017;4:e258-e271. doi: 10.1016/S2352-3026(17)30077-7
- Nascimento B, Callum J, Tien H, Peng H, Rizoli S, Karanicolas P, Alam A, Xiong W, Selby R, Garzon AM, et al. Fibrinogen in the initial resuscitation of severe trauma (FiiRST): a randomized feasibility trial. Br J Anaesth. 2016;117:775-782. doi: 10.1093/bja/aew343
|






