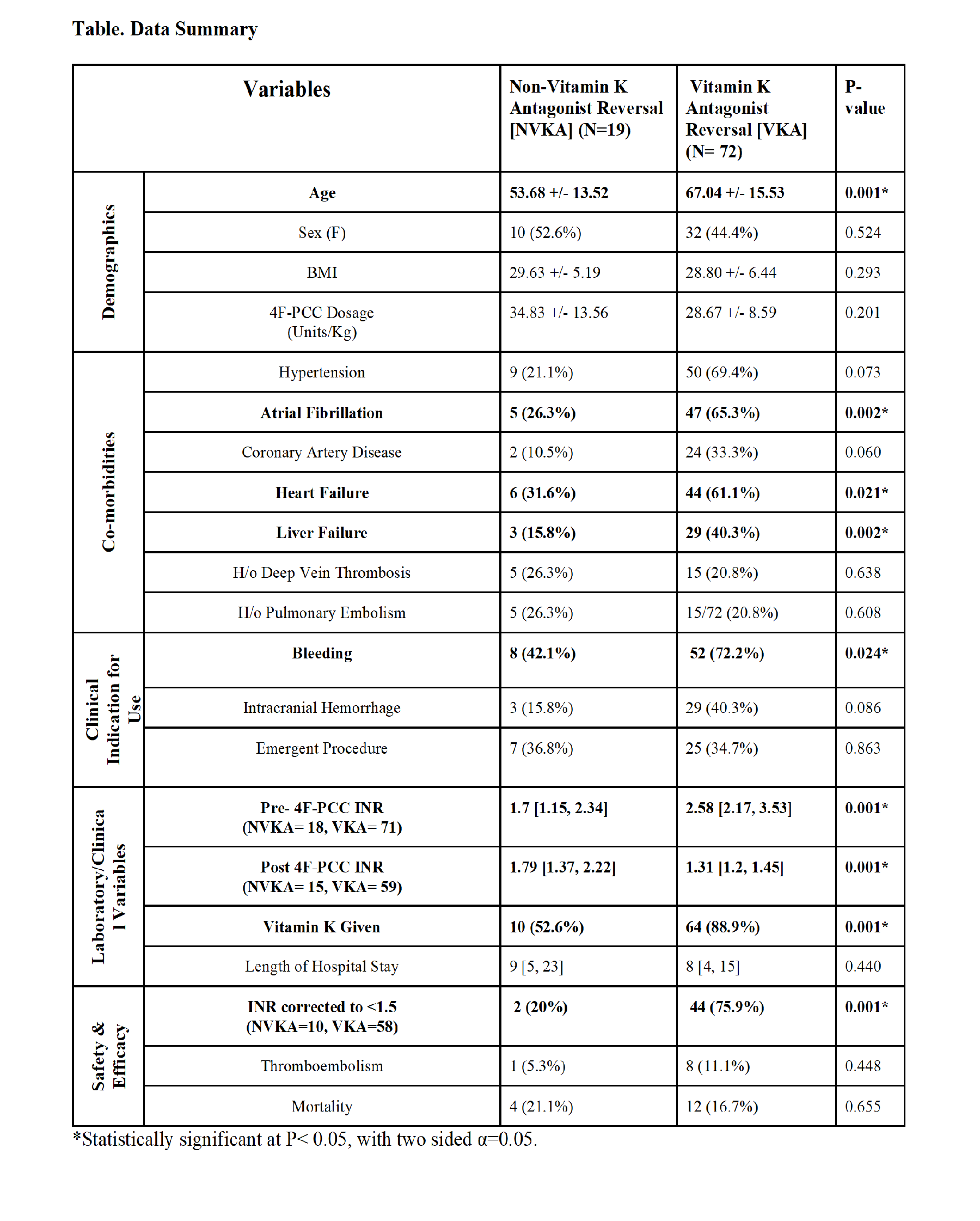
2017 Annual Meeting Abstract Winners - First PlaceSafety and Efficacy of 4-Factor Prothrombin Complex Concentrate in Patients Receiving Vitamin K Antagonists Compared to Patients not Receiving Vitamin K Antagonists: A Single Center Retrospective Review.Authors: V Vakayil, MBBS1, E Coler, PharmD2, J Larson, PharmD2, M Chandrashekar, BS1, A Lord, BS1, J Welbig, MLS(ASCP)SBB3, ND Zantek, MD, PhD4, JV Harmon, MD, PhD, FACS1 1University of Minnesota, Department of Surgery, Minneapolis, Minnesota, United States; 2University of Minnesota Medical Center, Department of Pharmacy, Minneapolis, Minnesota, United States; 3Fairview Health Services, Laboratory Administration, St. Paul, Minnesota, United States; 4 University of Minnesota, Department of Laboratory Medicine and Pathology, Minneapolis, Minnesota, United States Introduction A 4-factor prothrombin complex concentrate (4F-PCC) has been approved by the FDA for the reversal of vitamin K antagonists (VKA) in patients with major bleeding or need for an emergent surgery/invasive procedure. In patients with other causes of coagulopathy, 4F-PCC may improve hemostasis, however safety and efficacy of 4F-PCC in these patients has not been established. Methods A single center retrospective review of patients who received 4F-PCC from 2014-2016 was performed. Demographic, clinical, laboratory, safety, and efficacy variables were analyzed. Analysis was performed using SPSS (IBM, Armonk, NY). Data are presented as percent of evaluable patients or median [interquartile range] unless otherwise specified. Results 4F-PCC was administered to a total of 91 patients (46.2% female, 53.8% male) with 79.1% of patients receiving VKA therapy prior to administration. The indications for 4F-PCC were bleeding (48.4%), emergent surgery/procedure (17.6%), both bleeding and emergent surgery/procedure (17.6%), and other (16.5%). Liver failure was a frequent comorbidity in patients not receiving VKA [VKA (n=6) 8.3% versus no VKA (n=7) 36.8%, p=0.002] (ref. Table). International normalized ratio (INR) prior to 4F-PCC (pre-INR) was available for 97.8% of patients and was 2.4 [1.95, 3.28], with 78.7% of patients having a pre-INR >2.0. For patients with pre-INR ≥ 1.5, the first INR value following 4F-PCC infusion (post-INR) was analyzed. INR values drawn within 1 hour post-infusion (n=25) were 1.40[1.22, 1.58] and INR values drawn within 8 hours post-infusion (n=73) were 1.38[1.23, 1.55]. Among patients with a pre-INR ≥ 1.5 and a post-INR obtained within 8 hours, the post-INR correction (INR<1.5) rate was higher in patients previously on VKA therapy (VKA 75.9% versus no VKA 20.0%, p<0.001). No difference in mortality or thromboembolic complications was observed when comparing patients receiving versus not receiving VKA prior to 4F-PCC infusion. Discussion Patients not receiving VKA therapy had lower INR correction rates following 4F-PCC infusion but similar mortality and thromboembolic complication rates when compared to patients on VKA therapy. Further research on the use of 4F-PCC in patients not receiving VKA therapy, in particular patients with liver failure, should be pursued to establish the safety and efficacy in this population. |